Provides a standardized clinical evaluation of symptomatology associated with eating disorders
David M. Garner, PhD
Test for Eating Disorders with the EDI-3 and Help Determine Whether Patients Meet DSM-IV Diagnostic Criteria
A revision of one of the most widely used self-report measures of constructs shown to be clinically relevant in individuals with eating disorders, the EDI-3 includes enhancements that make the instrument more consistent with the psychological domains identified by modern theories to be most relevant.
Need to quickly screen? The EDI-3 Referral Form is an abbreviated version of the EDI-3 that quickly screens individuals for eating disorder risk.
Features and benefits
- This 91 item rating scale can be completed by clients age 13 to 53 years in only 20 minutes.
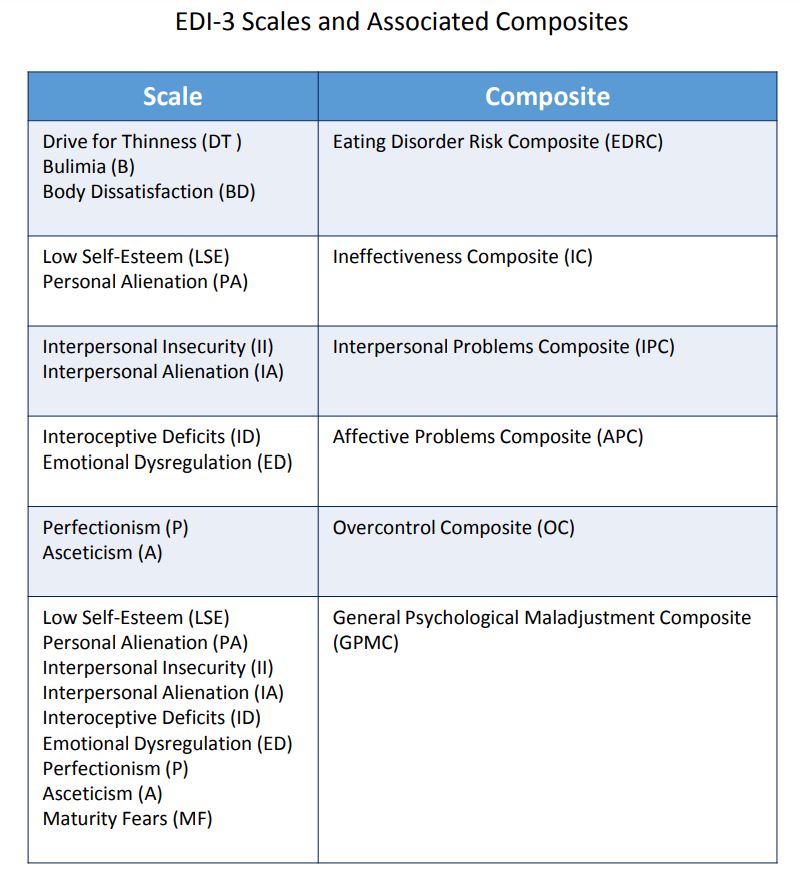
- Easily administered and scored, the EDI-3 yields
12 nonoverlapping scale scores and six composite
scores that can be used to create clinically meaningful profiles. The profiles can be linked to treatment plans, specific interventions, and treatment
monitoring.
- Provides data regarding frequency of symptoms (e.g., exercise patterns; use of laxatives, diet pills and diuretics; self-inducing vomiting) necessary to determine whether patients meet DSM-IV diagnostic criteria.
- Includes case studies.
- Includes clinical norms for adolescents in addition to U.S. and international adult clinical norms. It also provides multisite nonclinical comparison samples.
- Profiles can be linked to treatment plans, specific interventions, and treatment monitoring.
- Translated into 16 languages, including Arabic, Chinese, French, Russian, and Spanish.
- Administer and score online via PARiConnect.
Available in Spanish
The EDI-3 materials, including the manual, have been translated into European Spanish and designed especially for Spanish-speaking clinicians and their clients. Normative data were collected primarily in Spain, with additional data collected in some Latin American countries. Certain test items and stimuli in this translation vary from the English version because of cultural and linguistic differences between the countries and their language. Order the EDI-3 in Spanish.